-
1.
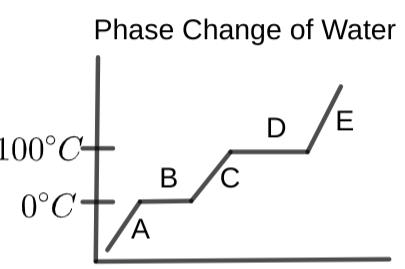
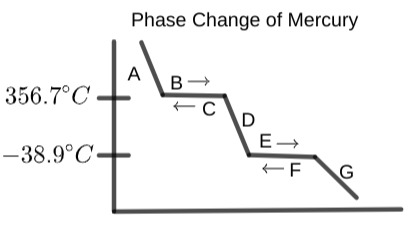
At the portion of the graph labeled "A", what state/process is occurring?

-
2.
What word best fits the portion of the graph labeled "C"?
-
3.
From the following, what word should be where the graph is labeled "E"?

-
4.
At the spot labeled "B", with the right point arrow, what state or process is occurring?

-
5.
What label should be placed at "D" (with the right point arrow)?

-
6.
"F" should have which of the following labels?

-
7.
Determine which word from below best describes "G" (left-pointing arrow):

-
8.
What should section "A" be labeled?

-
9.
Determine what is occurring in section "B" of the following graph:
-
10.
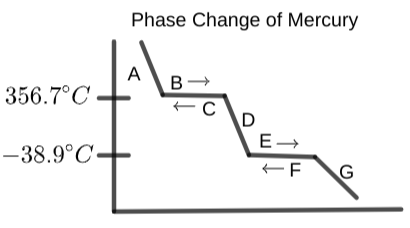
What word should replace the label "D" in the following graph?
-
11.
From the options below, label section "C" on the provided phase change diagram of mercury:

-
12.
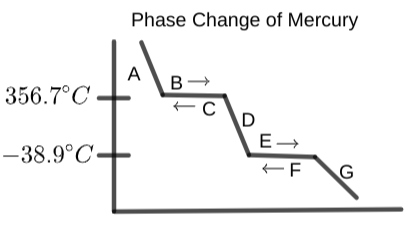
Select the word that best fits on region "E" on the graph below:
-
13.
What is occurring at section "F" on the phase change diagram?
-
14.
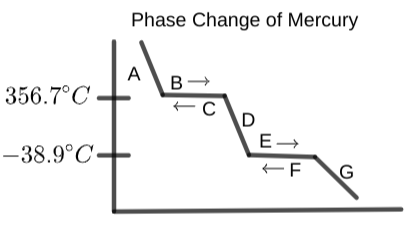
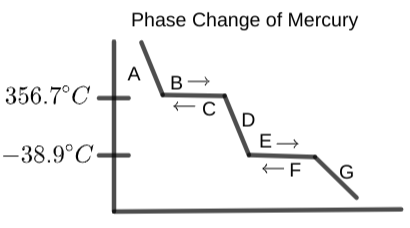
Determine the state of matter or phase change label that would best fit at region "G" on the provided diagram:
-
15.
Region "B" on the graph should have which of the following labels?

-
16.
What phase is found in region 1?

-
17.
What phase is found in region 2?

-
18.
What phase is found in region 3?

-
19.
What phase is found in region 4?

-
20.
What is point A known as?

-
21.
What is point B known as?

-
22.
If a substance transitions from region 1 to region 2, what process is happening?

-
23.
If a substance transitions from region 2 to region 3, what process is happening?

-
24.
Assume a substance transitions from region 3 to region 1. What process would that substance undergo?

-
25.
A substance in region 4 goes from 1000 bar to 1 bar of pressure. Assume the temperature does not change. What phase will the substance now be in?

-
26.
A substance undergoes a transition from region 3 to region 2. What phase change is taking place?

-
27.
A substance transitions from region 2 to region 1. What phase change would be observed?

-
28.
A substance is initially at the triple point. Assume that the temperature is increased to 350 K, and the pressure is increased to 10 bar. What will be the final phase of the substance?

-
29.
A substance is initially at the triple point. Assume the temperature is increased to 250 K, and the pressure is increased to 100 bar. What will the final phase of the substance be?

-
30.
A substance is initially at the critical point. Assume the temperature is decreased to 250 K, and the pressure is increased to 10,000 bar. What will the final phase of the substance be?
