Photons, Particle Soup & Nucleosynthesis
Table of Contents
- Cosmologists and Our Universe
- Photons and Particle Soup
- Cooling Off
- Nucleosynthesis
- Lesson Summary
- Learning Outcomes
One of the most difficult things to understand in astronomy is what happened at the very beginning of the Big Bang. No one really knows for certain what was going on at time zero, but cosmologists have an idea as to what happened in the fractions of a second thereafter. A cosmologist, by the way, is a physicist or astronomer involved in figuring out the origin, evolution, and properties of the universe.
So, in this lesson, you'll take a direct look at what cosmologists believe happened during the very first breaths of the universe's evolution.
In the first 10 millionth of a second after the Big Bang, the universe was filled with incredibly hot and dense high-energy photons. Photons are packets of electromagnetic energy traveling at the speed of light. You might be wondering what I mean when I say the photons were hot. To a cosmologist, hot means they have a spectrum equal to the blackbody radiation emitted by an object that has the same temperature.
Anyways, the photons in the early universe were actually gamma rays, which is a form of electromagnetic radiation, and consequently such photons were of very high energy because of the very short wavelength and high frequency nature of gamma rays.
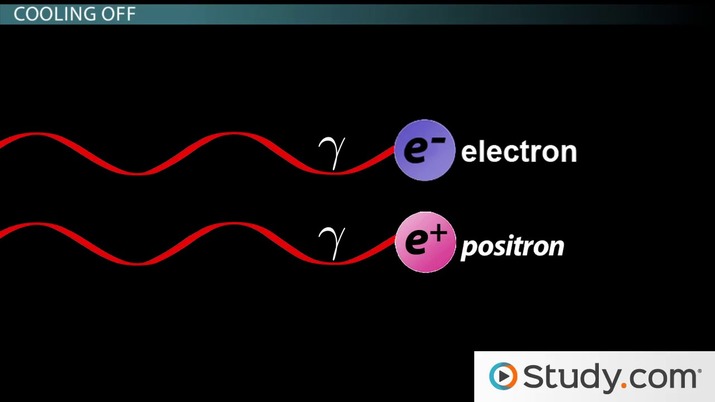
As a result of this high energy, two photons could collide to become two distinct particles in a process called pair production, the creation of a particle and an antiparticle from gamma rays. One particle that's created is normal matter, and the other particle is antimatter, a kind of matter, composed of antiparticles, which will annihilate matching ordinary matter on contact.
For example, normal matter would be in the form of an electron, while its antimatter particle would be a positive electron, the antiparticle of the electron, called a positron (sometimes also called an anti-electron). Similarly, there are protons and antiprotons, neutrons and anti-neutrons, and so on down the line.
In any case, one microsecond after the Big Bang, gamma ray photons had enough energy to make protons, neutrons, electrons, and their respective antiparticles. When such a particle and its antiparticle collide, they annihilate one another and convert their mass into energy, the energy of two gamma rays. This means the very early universe, at 1 microsecond in age, was a dynamic soup of matter and radiation moving from photons to particles to photons again.
As all of this was happening, the universe was actually expanding and the temperature of the radiation dropped as a result. The temperature drop caused the high energy of the photons to drop as well. This meant that the gamma rays no longer had enough energy to produce heavy particles like protons and neutrons.
Now, you'd think that as a result all the protons and neutrons would've simply combined with their antiparticles and destroyed themselves. This would mean there would be no matter and therefore no sun, no Earth, and no you. However, for some unknown reason, there was a small excess of normal particles. And thank your lucky stars, too! Because there would be no stars to thank if that wasn't the case. Antimatter is actually very rare nowadays.
While at about 0.0001 seconds, the gamma ray photons no longer had enough energy to make protons and neutrons, they still could make electrons and positrons because the latter two are way less massive than protons and neutrons. Just like you know it takes a lot of energy to produce something massive, like an ocean ship, you similarly know it takes far less energy to produce something a lot less massive, like a toy ship. Same thing here - the gamma ray photons had less energy, but still enough to make the less massive stuff, the electrons and positrons. But when the clock tolled one minute past the Big Bang, the ever-expanding universe had cooled so much that the photons no longer had enough energy to make electrons or positrons either!
As with the protons and neutrons, most of the electrons and positrons combined to form photons but, once again, a small excess (about one in a billion) of the electrons somehow survived. In the grand scheme of things, what all of this should mean to you is that all of the protons, neutrons, and electrons that make up our universe were made in the first minute after the Big Bang.
About two minutes after the Big Bang, the universe cooled down enough to allow for protons and neutrons to hook up and form deuterons, nuclei of deuterium (heavy hydrogen) consisting of a proton and a neutron. Meaning, a process of nucleosynthesis, the formation of new atomic nuclei, had begun.
Deuterons can easily react with protons in nuclear reactions in a stepwise fashion to eventually form ever more massive nuclei, namely helium and a tiny bit of lithium (and perhaps beryllium). You can think of this as taking a ball of playdough and mashing in another colored ball of playdough to make something more massive and new.
But by approximately minute 30, the universe had cooled so much, the nuclear reactions stopped entirely and, by mass, 75% of the early universe was thus made of hydrogen and the rest was almost entirely made of helium. Basically, the universe had become so cold that the playdough froze and you know you can't mash frozen playdough together! So, no new stuff was made anymore as a direct consequence of the Big Bang. This composition, mainly hydrogen and the rest helium, is what we know the oldest stars are made of, which makes sense since it's the only matter they had to work with in order to form.
Over a much longer period of time, these stars then produced other atoms inside of them, in a much slower version of nucleosynthesis, in order to make new elements with atomic weights greater than lithium that helped our sun, Earth, and your own body to eventually come into existence. Basically, the hot stars are like pots on a stove where ingredients like hydrogen and helium are combined to make new and tasty stuff.
This was a tough lesson, so let's cook up our own summary here to make things a bit easier for everyone. The first easy thing is the definition of a cosmologist, a physicist or astronomer involved in figuring out the origin, evolution, and properties of the universe. That's what this lesson was all about: the origin and evolution and properties of our universe right after the Big Bang.
In the beginning, the universe was filled with hot and dense photons. Photons are packets of electromagnetic energy traveling at the speed of light. They were high-energy gamma ray photons that crashed together to become two distinct particles in a process called pair production, the creation of a particle and an antiparticle from gamma rays.
One particle that's created is normal matter, and the other particle is antimatter, a kind of matter, composed of antiparticles, which will annihilate matching ordinary matter on contact. For example, normal matter would be in the form of an electron, while its antimatter particle would be a positive electron, the antiparticle of the electron, called a positron (sometimes also called an anti-electron).
As the universe expanded and cooled thereafter, protons and neutrons couldn't be made anymore because the gamma ray photons didn't have enough energy. Eventually, no more electrons could be made either for the same reason.
The universe finally cooled down enough where deuterons, nuclei of deuterium (heavy hydrogen) consisting of a proton and a neutron, could form. Deuterons could then combine with protons to initiate nuclear reactions that formed heavier atomic nuclei, like helium and lithium, in a process known as nucleosynthesis.
The rest of the elements, heavier than lithium, were made later inside of stars in a slower, pressure-cooker-like version of nucleosynthesis.
You will have the ability to do the following after watching this video lesson:
- Define cosmologist, photons, antimatter, positron and deuteron
- Describe the processes of pair production and nucleosynthesis
- Explain what happened after the Big Bang using these processes and the cooling off period in between them
Register to view this lesson
Unlock Your Education
Become a Study.com member and start learning now.
Become a MemberAlready a member? Log In
BackResources created by teachers for teachers
I would definitely recommend Study.com to my colleagues. It’s like a teacher waved a magic wand and did the work for me. I feel like it’s a lifeline.