David has taught Honors Physics, AP Physics, IB Physics and general science courses. He has a Masters in Education, and a Bachelors in Physics.
Thermal Expansion & Heat Transfer
Table of Contents
- What Is Heat Transfer?
- Rate of Heat Transfer
- Thermal Expansion
- Example Calculation
- Lesson Summary
- Learning Outcomes
Heat energy, or thermal energy, is the energy of a substance or system in terms of the motion or vibrations of its molecules. The faster the molecules in a substance move, the more heat energy they have. Heat energy can transfer from one object to another by three methods: conduction, convection, and radiation.
Conduction is where heat is transferred between two objects due to physical contact between them. The molecules literally hit each other, causing energy to transfer. Convection is where a hot part of a substance rises due to its lower density, while cooler parts sink, creating cycles that transfer heat upwards. And radiation is where heat is transferred through electromagnetic waves, such as the radiation from the sun.
The rate of heat transfer can be calculated, but there are separate equations for the three types of heat transfer. There's an equation to calculate heat transfer by conduction, one for convection, and one for radiation. But today we're just going to focus on conduction. The rate of heat transfer by conduction varies according to several factors: the thickness of the material through which it's transferring, the length of the material, and the temperature difference at the two ends of the material. In equation form, it looks like this:
(Q / t) = [kA(delta T)] / d
Here, Q over t is the heat transfer per second, measured in Joules per second, Q is the amount of heat energy (Joules), and t is the time in seconds. K (or to be exact, the Greek letter kappa) is the thermal conductivity of the material in watts per meter degrees C. A is the surface area of the material, d is the thickness of the material, and delta T is the difference in temperature between the two ends of the material, measured in either Celsius or Kelvin. Delta T is equal to the difference between the hottest temperature and the coldest.
Our understanding of thermal expansion is vitally important. It allows us to measure the temperature, stop bridges from collapsing, and open stubborn jars. Thermal expansion is where materials expand while being heated, causing them to take up more space. Some materials expand more than others - metals expand a lot, for example. But, in general, materials only expand due to the motion of their molecules. When something gets hotter, its molecules move faster. This is, after all, what temperature is: the average kinetic energy of the molecules in a substance. Molecules that move faster use more space. So, materials contract when they cool, and they expand when you heat them up.
Understanding thermal expansion is extremely useful. The material inside a thermometer expands when it gets hotter, moving it up the carefully calibrated temperature gauge to tell you how hot it is. Bridges have so-called expansion joints, which are metal jagged teeth that allow the bridge to expand on hot days and contract on cold days. Without these expansion joints, bridges would collapse! And that would be a big mess to clean up, so nobody wants that.
And then there are tight jars in the kitchen. How can you open a tight jar? Well, there are a lot of ways. You can break the seal by knocking it against a counter-top, or you can grip it with a piece of rubber. But another way is to use the power of thermal expansion. Run the metal jar lid under hot water, and the jar will expand allowing you to open it. But hold on a minute, wouldn't making the metal expand cause it to tighten, making it even harder to open?
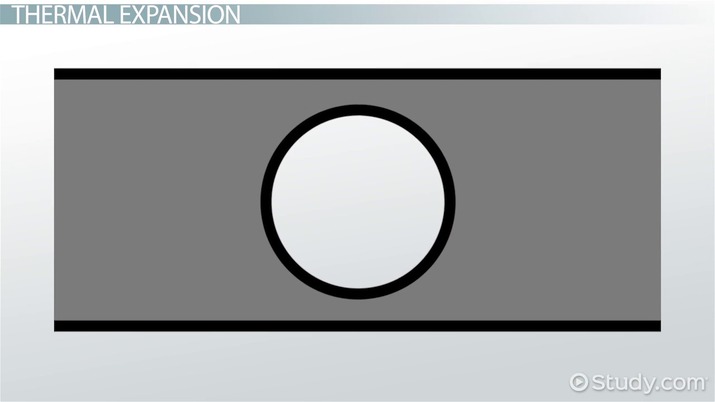
That tends to be how people think, but the problem is we're not thinking carefully about how objects expand. Let's say you have a piece of metal with a hole in the middle. If you expand the piece of metal, does the hole get bigger or smaller? It's easy to imagine the metal expanding to fill the hole, but that's not what happens. If you take a graphic like this, and expand it on a computer, the whole shape gets larger, including the hole (please see the video beginning at 03:40 to see this illustration). So, heating up a jar lid increases the size of the gaps between the lid and the glass, making it easy to open.
Okay, let's go back to the conduction equation and go through an example. Heat is being transferred through a metal of thermal conductivity 60 Watts per meter degrees C. The material has a surface area of 0.1 meters squared and a thickness of 0.05 meters. If one end of the material has a temperature of 20 degrees C and the other end has a temperature of 100 degrees C, what is the rate of heat transfer across the material? And how much heat will transfer in 42 seconds?
First of all, we should write down what we know. The thermal conductivity, kappa, is 60, the surface area A is 0.1, and the thickness d is 0.05. We can also find delta T by finding the difference in temperature between the two ends, which is 100 minus 20, which equals 80. We also have a time, t, of 42 seconds, but that's part of the second question.
The first question asks us to find the rate of heat transfer, which is Q over t, so all we have to do is plug numbers in and solve for Q over t. When we do that, we get a value of 9,600 Joules per second.
Then, the second question wants to know how much heat transfers in 42 seconds. Well, if 9,600 Joules of heat transfer every second, and we want to know how much transfers in 42 seconds, we just multiply. Multiplying 42 by 9,600 we get 403,200 Joules. Or, in other words, this is like solving for Q in the original equation, instead of Q over t you multiply both sides of the equation by t.
Heat energy, or thermal energy is the energy of a substance or system in terms of the motion or vibrations of its molecules. The faster the molecules in a substance move, the more heat energy they have. Heat energy can transfer from one object to another by three methods: conduction, convection, and radiation.
Conduction is where heat is transferred between two objects due to the physical contact between them. The molecules literally hit each other, causing heat to transfer. Convection is where the hot part of a substance rises due to its lower density, while cooler parts sink, creating cycles that transfer heat upwards. And radiation is where heat is transferred through electromagnetic waves, such as the radiation from the sun.
The rate of heat transfer can be calculated, but there are separate equations for the three types of heat transfer. The rate of heat transfer by conduction in equation form looks like this:
(Q / t) = [kA(delta T)] / d
Here, Q over t is the heat transfer per second, measured in Joules per second, Q is the amount of heat energy (Joules), and t is the time in seconds. K (or to be exact, the Greek letter kappa) is the thermal conductivity of the material in Watts per meter degrees C. A is the surface area of the material, d is the thickness of the material, and delta T is the difference in temperature between the two ends of the material, measured in either Celsius or Kelvin. Delta T is equal to the difference between the hottest temperature and the coldest.
Thermal expansion is where materials expand while being heated, causing them to take up more space. Some materials expand more than others - metals expand a lot, for example. But, in general, materials only expand due to the motion of their molecules. When something gets hotter, its molecules move faster. This is, after all, what temperature is: the average kinetic energy of the molecules in a substance. Molecules that move faster use more space. So, materials contract when they cool, and they expand when you heat them up. When you expand a material, the whole shape expands - the shape stays the same, but the size increases. So, both the material and the gaps in the material expand at the same rate.
Register to view this lesson
Unlock Your Education
Become a Study.com member and start learning now.
Become a MemberAlready a member? Log In
BackResources created by teachers for teachers
I would definitely recommend Study.com to my colleagues. It’s like a teacher waved a magic wand and did the work for me. I feel like it’s a lifeline.