Zymogen | Definition, Activation & Granules
Table of Contents
ShowWhat are zymogens in biology?
Zymogens are proenzymes (i.e., inactive enzymes). Any protein that contains an inactive enzyme or protein until a portion of the protein is removed can be considered a zymogen.
Which enzymes are secreted as zymogens?
In general, destructive catalytic enzymes are secreted as zymogens. They do this so that they do not destroy the cell producing them (e.g., proteases, lipases, pore-forming subunits, etc.)
Which cells have zymogen granules?
Zymogen granules are primarily found in pancreatic acinar cells and salivary glands within the mouth. Both cells package catabolic enzymes as zymogens in these granules for secretion in response to hormonal signals.
What is the function of zymogen?
The function of a zymogen is to keep the enzyme contained within it inactive. As zymogens are generally catabolic, this keeps the enzyme from destroying the cell producing it. Once secreted from the cell, the zymogen is cleaved, creating the active enzyme.
Table of Contents
ShowA zymogen also termed a proenzyme, is a dormant enzyme activated when a portion of the protein is cleaved, either chemically/or enzymatically. Zymogens contain enzymes that, when active, have a wide variety of functions, including proteolysis, lipolysis, receptor ligands (i.e., hormones), protease inhibition, pore-formation in membranes, blood coagulation, and others. Zymogens are found in all living organisms, and many zymogens regulate their own active form (i.e., creating a feedback loop).
Zymogens allow the cell to control when and where an enzyme becomes active, a critical step in the evolution of multicellular organisms. The importance of controlling where enzymes get activated is exemplified by gastric zymogens, which generally have protease function once activated. If the active form of trypsinogen, the protease trypsin, were to be active inside the pancreatic acinar cells that release it, this would damage and lead to the destruction of the acinar cells. Indeed, most, if not all, secretory cells in the body would be destroyed by the enzymes they secrete if the active enzyme did not have a proenzyme state inside the cell that produces it.
Examples of Zymogen
What are some zymogen examples? Zymogens are produced by secretory cells throughout the body and within a number of immune cells and likely within cell populations we have yet to identify. A list containing a variety of zymogens and the functions their enzymes can perform once activated is provided here. This list is not exhaustive but will provide a foundation.
- Trypsinogen, chymotrypsinogen, and pepsinogen are zymogens for the gastric proteases trypsin, chymotrypsin, and pepsin.
- Angiotensin is the zymogen for the hormone angiotensin II, a major regulator of blood pressure and the secretion of other hormones.
- Prothrombins the zymogen for thrombin, a key component of blood clotting.
- Prolipases are the zymogens for some bacterial lipases.
- The C1 complex in the complement system is comprised of at least five different zymogens that, when activated, combine to form pores in the membranes of pathogenic cells.
 |
Zymogen activation is the process by which a proenzyme is converted into its active form. A protease activates most zymogens via proteolysis (i.e., the zymogen protein is cleaved). Proteases bind to specific protein sequences and then cleave the amino-acid backbone of the target protein. In the case of zymogens, this cleavage allows the protein to adopt a new conformation (i.e., structure), which is now capable of enzymatic activity.
Trypsinogen is produced in the acinar cells of the pancreas and secreted into bile. The bile contains a plethora of digestive enzymes which are transported to the small intestine. Trypsinogen is cleaved by enteropeptidase, secreted from the brush border cells in the small intestine. Trypsinogen cleavage by enteropeptidase creates two fragments, active trypsin, and trypsin-active-peptide (TAP). Trypsin is a protease and stays in the small intestine to continue the process of protein degradation necessary for nutrient absorption in the gut. At the same time, TAP is actively removed from the body through urination. TAP levels in the urine provide a diagnostic for measuring trypsin activity in the gut.
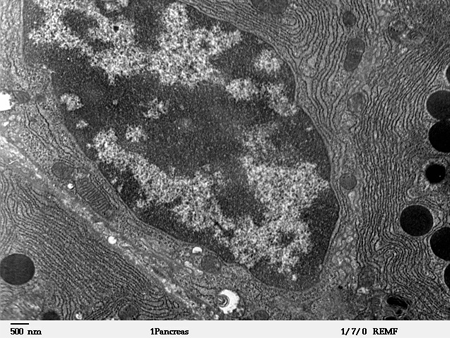
What are zymogen cells? As with everything in the body, the secretion of gastric enzymes by the pancreas is tightly regulated and highly specific (i.e., the enzymes that are needed by the small intestine are the ones that are secreted). This process is accomplished by zymogen granules in pancreatic acinar cells. Zymogen granules are vesicles created by the Golgi apparatus in cells. Vesicles in the Golgi are packaged with zymogens and released as pre-granules through a mechanism that has yet to be fully elucidated. Pre-granules can fuse to form mature zymogen granules. The vesicles are curated during the maturation process for the cargo (i.e., zymogens) they contain.
Exactly how the cargo of zymogen granules is curated remains unknown. However, zymogen vesicles contain a wide variety of proteins in their membranes; these include ion channels, vesicle trafficking proteins, proteases, small GTPases, and others which allow the vesicle to be filled with the correct cargo and transported to the plasma membrane. The zymogen granules create a pool of readily-releasable vesicles at the plasma membrane. When the acinar cells receive hormonal messages from the small intestine, the zymogen granules undergo exocytosis and fuse with the plasma membrane, releasing the zymogens into the bile.
Pepsinogen is the zymogen for the proteolytic enzyme pepsin. Pepsin is an endopeptidase that breaks down proteins into smaller and smaller peptides. Pepsin is an important protease in the stomach as it has a preference for cleaving at aromatic amino acids. Unlike trypsinogen, pepsinogen is not directly activated by another protease; it needs a little help from a few protons. Pepsinogen is manufactured by chief cells, which then secrete it into the stomach. Once in the stomach, the low pH (i.e., a high level of free protons) in the stomach initiates a conformational change in the structure of pepsinogen, exposing a pepsin cleavage site. Active pepsin that is already present in the stomach cleaves pepsinogen to create a new pepsin enzyme. This process is a prime example of zymogen activation by the active enzyme it produces.
 |
Zymogens are proteins that contain an inactive enzyme (i.e., a proenzyme). Zymogens are an important part of physiology as they enable the production of inactive enzymes within the cell that are not activated until after they are secreted. Many zymogens are catabolic enzymes that degrade proteins, lipids, and sugars, and they would damage the cell manufacturing them if they were produced as active enzymes. Proenzymes within zymogens are activated by cleaving the zymogen, releasing the active enzyme.
Zymogen granules in secretory cells, such as acinar cells in the pancreas, are vesicles containing zymogens ready for release. Upon hormonal stimulation of the acinar cells, zymogen granules fuse with the plasma membrane and are released into the extracellular fluid, in this case, bile, and transported to the small intestine for processing into active enzymes. Studies to determine the full composition of zymogen granules and the mechanisms by which zymogen granules are packaged are ongoing.
Video Transcript
What Is a Zymogen?
Have you ever eaten a candy bar? Can you just pop it in your mouth? No, because first you have to remove the wrapper.
A zymogen is like a wrapped candy bar. In order to get to the good stuff, you need to tear away what's keeping you from it. Zymogens, or proenzymes, are enzymes that aren't functioning yet because their action is blocked by a ''wrapper.'' The ''wrapper'' can be a link between two amino acids, the building blocks of proteins, like a piece of string keeping a box closed. Or it can be an extra section of protein, like a jar lid.
But what are enzymes? Enzymes are proteins that help chemical reactions happen faster via special places called active sites. Imagine you want to make a fruit smoothie. You could hand-mush the fruit or put the fruit in a blender. The blender is like an enzyme; the active site is like the blades of the blender. The fruit becomes a fruit smoothie faster in the blender than if you squish it by hand.
Enzymes help make many things in the cell, but they can also unmake them. Enzymes that chop up proteins are called proteases. When cells make enzymes, especially proteases, they often make them as zymogen, an inactive form of the enzyme. This is so they don't go crazy and are only used when needed. Imagine your reaction if your blender suddenly hopped about on the counter, out of control, spewing half-chopped fruit everywhere. The counter would be a mess, and so would the cell.
Zymogens also ensure the enzyme folds properly (has the correct 3-D form), is stable in unfavorable environments, and goes to the proper place so it functions where it's supposed to.
You can recognize most zymogens by their name. Enzymes that begin with ''pro-'' or end with ''-gen'' are often the zymogen form. For example, prothrombin is the zymogen form of thrombin, an enzyme involved in blood clotting. Pepsinogen is the zymogen form of pepsin, the enzyme found in your stomach that helps digest food.
Zymogen Activation
Zymogens are activated by snipping the bonds between two or more amino acids, rather like cutting a balloon string so that it floats away. When the bonds are cut, the enzyme changes its conformation, its 3-D structure, so that the active site is free and able to become active. Upon activation, sometimes pieces of the protein completely leave the enzyme, like taking the wrapper off a candy bar. Other times, the pieces of protein fold in and become part of the enzyme, like a catapult being pulled back.
Zymogens can be activated by proteases that cut the amino acid bonds. They can also be activated by the environment and become autocatalytic. Autocatalysis is self-activation and happens when something in the environment allows the zymogen to cut its own chemical bonds. Pepsinogen, for example, does not become pepsin until the pH is around 2 to 3. The extra hydrogens found in the lower pH make the molecule cut its own bonds that are preventing it from functioning as a digestive enzyme.
Zymogen Granules
Inside the cells of your pancreas and salivary glands are proteases that can activate digestive enzymes. To make sure the proteases inside the cell can't change into the zymogen form before they are released into your digestive system, the cell uses special holding rooms called granules.
Zymogen granules are places in the cell that keep zymogens safe from the proteases inside the cell. They are like little rooms, or little bubbles, full of different types of zymogens. They are mostly found in acinar cells found in the pancreas and salivary glands that group together like the bumps on a raspberry.
Most zymogen granules begin formation where the zymogens are first formed: the endoplasmic reticulum (ER). The ER is like the cell's factory; it's where proteins are made. From there, they are sent to the Golgi. The Golgi is like the cell's post office; it's where finished products are packed and shipped to different parts of the cell. From the Golgi, fully formed zymogen granules begin to gather at the apex, or the top of the cell.
Once there, the granules wait like excited kids ready for the signal to begin an Easter egg hunt. The ''On your mark . . .'' signal is given when special molecules bind to specific receptors on the cell's membrane. The ''Get set . . .'' signal involves ATP (adenosine triphosphate), as granules attach to and gather on the inside of the cell's membrane. Both calcium and cAMP (cyclic adenosine monophosphate) are involved in the ''Go!'' signal.
At the ''Go!'' signal, the granules fuse, or become one, with the cell's membrane. The zymogens are then released outside the cell and make their way to the digestive tract where they are activated and begin digestion. The whole process of zymogen granule movement from inside the cell to their release outside the cell is called exocytosis.
If zymogen granules are activated inside the cell, the digestive enzymes start to act inside the cell, and a disease condition called pancreatitis develops. Pancreatitis is an inflammation of the pancreas that causes pain in the stomach area and can happen fast (acute) or over time (chronic). If the inflammation is severe, it can be life-threatening.
Lesson Summary
Enzymes are proteins with active sites that help speed up chemical reactions. Proteases are enzymes that cut up other proteins and, because of their destructive properties, are often made by the cell as zymogens. Zymogens, or proenzymes, are inactive forms of enzymes that aid in enzyme folding, stability, and targeting. Zymogens can be activated by proteases or by their environment autocatalytically (self-activation). Activation involves a conformational (3-D structure) change in the protein that frees the active site to function.
Digestive enzymes are made as zymogens and are stored in zymogen granules, places in the cell that keep zymogens safe from proteases. Zymogen granules can be found in the acinar cells of the pancreas and salivary glands. They release zymogens outside the cell and into the body by a process called exocytosis. If zymogen granules are activated inside the cell, they can cause pancreatitis, an inflammation of the pancreas that causes pain in the stomach area.
Medical Disclaimer: The information on this site is for your information only and is not a substitute for professional medical advice.
Register to view this lesson
Unlock Your Education
Become a Study.com member and start learning now.
Become a MemberAlready a member? Log In
BackResources created by teachers for teachers
I would definitely recommend Study.com to my colleagues. It’s like a teacher waved a magic wand and did the work for me. I feel like it’s a lifeline.






